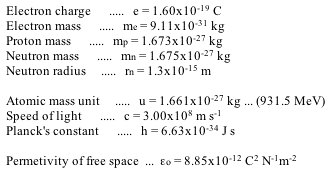Calculations - Test 2 - IB2
Name .................................
Constants
1a Write down the maximum energy of electrons as they
strike the anode in a cathode ray tube in electron Volts when
the anode voltage is 4500 Volts.
b Convert the maximum electron energy to Joules.
c Find the maximum velocity of electrons in the cathode ray tube as they reach the anode by using the kinetic energy relationship.
2 Supposing the particle in the tube was a positive electron and the anode were made from tungsten with an atomic number of 79. Calculate the distance of closest approach of the positive electron in a head on collision with the stationary tungsten nucleus.
The potential near a large positive point charge is given by ...
The kinetic energy of the electron in Joules as it strikes the anode has been calculated in part b above.
3 In Bohr's model of the hydrogen atom the electron is imagined to orbit the nucleus with a restricted set of angular momenta.
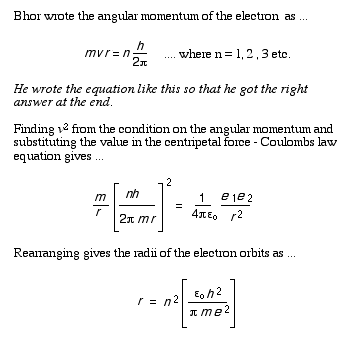
... where n is an integer that runs from 1, 2 upwards.
a Find the radius of the lowest energy electron orbit in meters.
b Convert this radius nanometers.
c Calculate the radii of the lowest four energy levels in nanometers [n = 1, 2, 3 and 4] and sketch an orbital diagram of the hydrogen atom.