Measurements and Their Uncertainties
It is important to report all measurements in science appropriately. This means that the number should be rounded to correctly represent the level of precision. The uncertainty in the measurement should also reported. Here are a number of examples of measurements, with explanations of how they should be reported.
| 1. A student uses a meter stick to measure the height of a table. He is able to make an accurate measurement of the height. He measures the height of the table to be 78.0 cm. He is confident that his measurement is accurate within 0.1 cm. He should report the height of the table as 78.0 ± 0.1 cm.
If he reports the height as 78 cm, it means that he rounded the height to the nearest centimeter, which is sloppy. If he reports the height as 78.00 cm, it means that he's claiming he knows the height to the nearest hundredth of a centimeter, which is a lie. A meterstick can measure to the nearest tenth of a centimeter, so the height should be reported as 78.0 ± 0.1 cm.
|
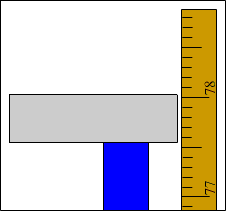 Example 1: The height is 78.0 ± 0.1 cm |
2. A student uses a meter stick to measure the height from which she is dropping a ball. She measures the height as 40.0 cm. But it is difficult to hold the ball steady as it is dropped. She therefore is only confident that the ball was within 0.5 cm of the measured height when it was released. She should report the height as 40.0 ± 0.5 cm.
|
|
3. A student measures the mass of a piece of potato on a digital mass balance to be 3.27 grams. All digital instruments are assumed to have an uncertainty of one unit in the last decimal place. She should report the mass as 3.27 ± 0.01 g.
|
|
4. A student measures the temperature of a beaker of water with a thermometer. The thermometer scale has markings every 1 degree celsius, but the markings are far enough apart that he can estimate the temperature 27.7 °C. He is confident that his estimate is off by no more that 0.2 °C. He should report the temperature as 27.7 ± 0.2 °C. |
