Sample Lab Activity Notes
Example 1
A Lab Activity Note should be short and succinct, while giving all the information needed to understand what was done and what the results show. Notice that each section is very short, so section subtitles are not included. Notice also that the purpose of the activity is presented as the title of the Note.
The Reduction of Copper Oxide to Copper
Using Carbon as the Reducing AgentMany metal ores (for example iron ore Fe2O3) can be reduced to the metal by reaction with carbon at high temperatures. The reduction of iron ore requires a temperature of 1200°C. Copper oxide reduction can be achieved with a lower temperature of about 600°C.
Fine black powdered carbon and copper oxide were mixed in a test tube. The tube was heated as strongly as possible with a butane flame for 5-10 minutes until the glass became distorted and copper metal was formed (see figure 1).
The chemical reaction can be summarized as ...

The black copper oxide is reduced to copper metal, by the reaction with carbon, the reducing agent.
Example 2
Note that each section is long enough to justify including subtitles. Notice also that the procedure is included as an attachment.
Observing Leaf Stomata
Introduction:
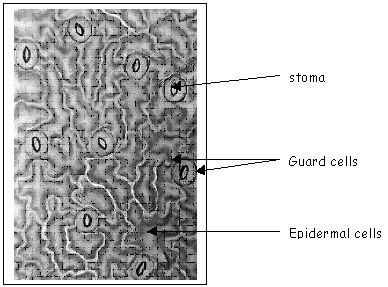
Photosynthesis is the process in which green plants use energy from the sun to convert carbon dioxide from the air and water from the plant cells to make glucose, which the plant uses as food, and oxygen gas, which is given off to the atmosphere.The carbon dioxide and oxygen gets into and out of the leaf through small pores on the bottom of the leaf called stomata. The actual opening into the leaf is called a stoma, and the two cells that can change shape to open and close the stoma are called guard cells.
In this activity, we are going to make a nail polish impression of leaf stomata and observe the stomates under high power magnification. The stomate density (how many stomates are in every square millimeter) can be calculated using the formula:
Stomate density = number of stomates in high power view / 0.2 mm
Materials and Procedure:
Refer to Annex A, class handout “Observing Leaf Stomata”
Data Collection and Analysis:
Below is a picture taken from the microscope digital camera of our leaf impression at 400 x magnification.
We observed 9 stomata in our field of view. The stomata density using the formula given was calculated to be 45 stomates per mm2.
Conclusion:
The method of obtaining a stomata impression from nail polish and tape worked very well. It was very easy to see the stomates. A stomata density of 45 stomates/mm2 was quite large. It makes sense that there would be a lot of stomates in the leaf, since the leaf is designed to perform photosynthesis. If there weren’t enough stomates, then the carbon dioxide and oxygen gases would not be able to get into or out of the leaf very well. It would be interesting to compare stomata density with other leaf species, or leaves that are in the shade versus the direct sun.